Annals of Surgery on X: "Patient-Reported Adverse Events during Neoadjuvant Therapy in a Phase 2 Borderline Resectable Pancreatic Cancer Clinical Trial (Alliance A021501) #AmerSurg23 https://t.co/zoJ0tFPrHB" / X

Feasibility of Implementing the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events in a Multicenter Trial: NCCTG N1048 | Journal of Clinical Oncology
Development and Testing of the Pediatric Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events

Distribution of most severe adverse events (CTCAE Grade 4-5) on patient... | Download Scientific Diagram
![PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/72d6066aa43136e48ae6226e3dba7e542a7a02de/5-Table1-1.png)
PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar
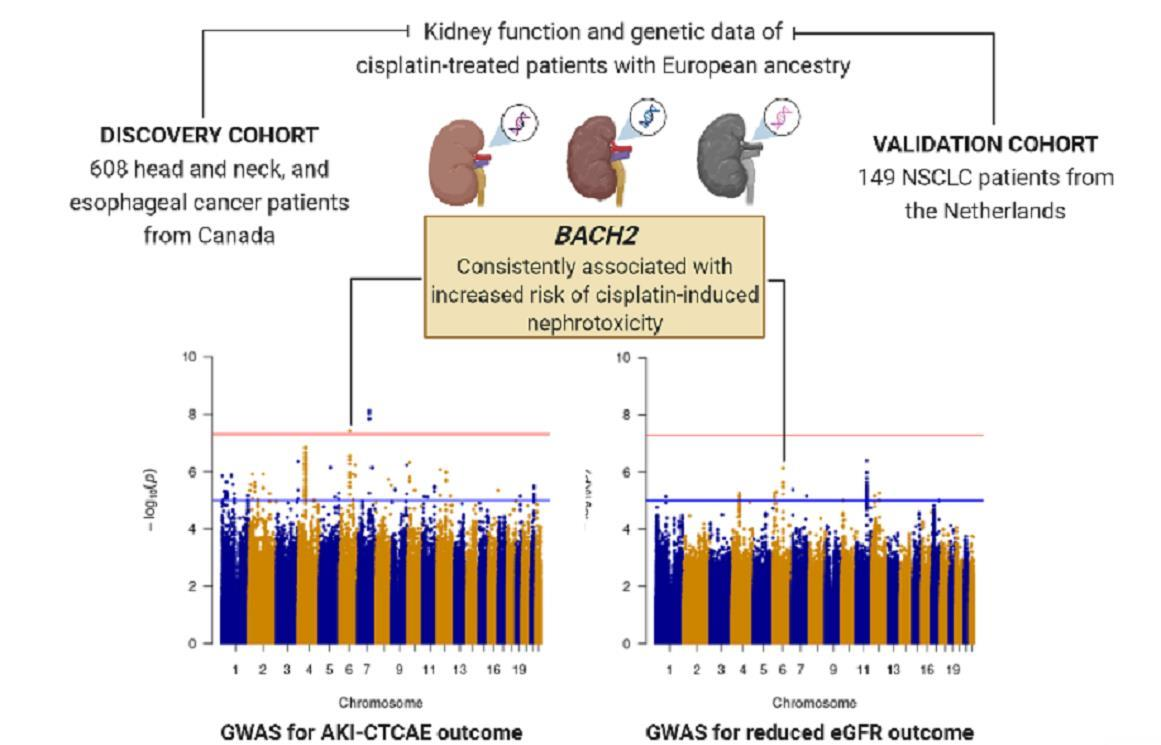
JPM | Free Full-Text | Association between Genetic Variants and Cisplatin-Induced Nephrotoxicity: A Genome-Wide Approach and Validation Study

Evaluation of the patient experience of symptomatic adverse events on Phase I clinical trials using PRO-CTCAE | British Journal of Cancer
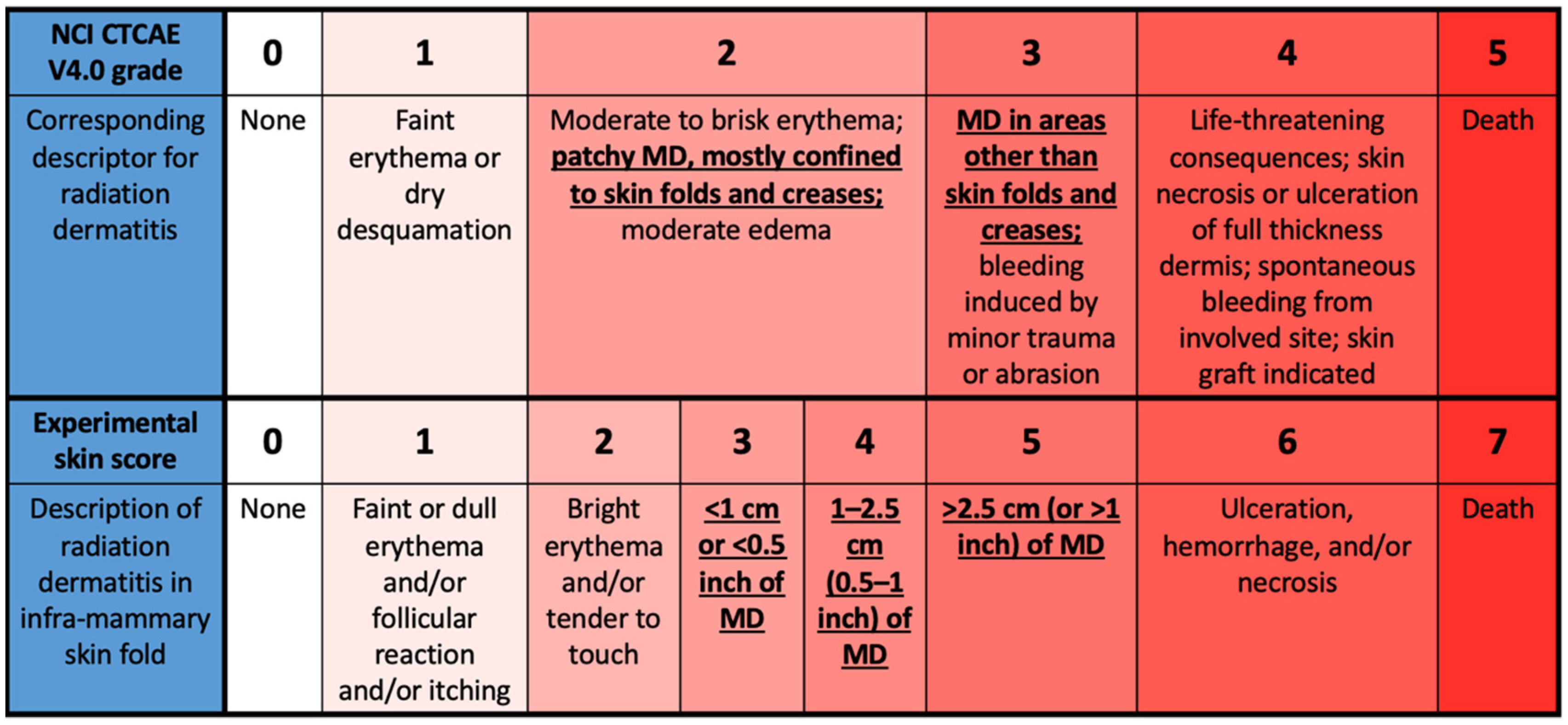
Current Oncology | Free Full-Text | Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients

A tailored phase I-specific patient-reported outcome (PRO) survey to capture the patient experience of symptomatic adverse events | British Journal of Cancer

Feasibility of Implementing the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events in a Multicenter Trial: NCCTG N1048 | Journal of Clinical Oncology

Feasibility of Implementing the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events in a Multicenter Trial: NCCTG N1048 | Journal of Clinical Oncology



![Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF](https://image.slidesharecdn.com/ctcae4-022009-09-15quickreference8-5x111-120711021618-phpapp01/85/ctcae-402-20090915quickreference85x111-2-320.jpg?cb=1668437655)


![Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF](https://image.slidesharecdn.com/ctcae4-022009-09-15quickreference8-5x111-120711021618-phpapp01/85/ctcae-402-20090915quickreference85x111-3-320.jpg?cb=1668437655)